
The modernization of a universities General Chemistry laboratory program can be a daunting and yet necessary task. This is especially true when you have 2000+ students taking General Chemistry every year. The University of Tennessee, Knoxville decided it was time to modernize their General Chemistry Lab Program for them to remain competitive. They knew that Electronic Data Collection Technology needed part of this process, and needed to be integrated into the labs. I am happy to say The University of Tennessee, Knoxville decided MeasureNet's Electronic Data Collection System was the technology selected in the redesign of their General Chemistry Laboratory Program.
“The laboratory makeover not only benefits the first-year students, but will help improve their preparation and success as they go on to take upper-level chemistry courses, “
Al Hazari, Director of Undergraduate Laboratories.
%20Systems-resized-601.png)
Department of Chemistry and Biochemistry, Miami University, Oxford, Ohio 45056, United States
J. Chem. Educ., 2013, 90 (4), pp 500–505
DOI: 10.1021/ed300340x
Publication Date (Web): March 15, 2013
Copyright © 2013 The American Chemical Society and Division of Chemical Education, Inc.
Abstract
The large class sizes of first-year chemistry labs makes it challenging to provide students with hands-on access to instrumentation because the number of students typically far exceeds the number of research-grade instruments available to collect data. Multifunctional chemical analysis (MCA) systems provide a viable alternative for large-scale instruction while supporting a hands-on approach to more advanced instrumentation. This study describes how the capabilities of MCA systems are extended to introduce liquid chromatography (LC) and flow injection analysis (FIA) in undergraduate laboratories. A semi-micro plastic cuvette with a Teflon tubing insert is fashioned as the flow cell for a MCA absorbance–fluorescence detector. Two MCA systems, Vernier and MeasureNet, are used in two unique experiments demonstrating the detection of salicylate in aspirin tablets by FIA and the LC separation of a mixture of riboflavin and fluorescein. Both instruments, composed of a syringe pump, T-injection valve, and the MCA detector, operated in the kinetic mode, are rugged and inexpensive permitting student construction, if desired.
Micro Solid-Contact Ion-Selective Electrode Using a Carbon
Nanotube Tower as Ion-to-Electron Transducer and Conductive
Substrate
Xuefei Guo, Timothy Meyung, Yeoheung Yun, Vesselin N. Shanov, H. Brian Halsall, William
R. Heineman
Electroanalysis Volume 24, Issue 11 DOI: 10.1002/elan.201200348
Abstract
Solid contact (SC) ion-selective electrodes (ISEs) have been recognized as the next generation of ISEs. In this work, the electrical conductivity and mechanical strength of a carbon nanotube (CNT) tower enable it to play the dual roles of transducer and substrate for micro SC-ISEs. The electrode had a close to Nernstian slope of 35 mV/decade aCa2+, a linear range of four orders of magnitude of calcium ion activity (10�-5.6 to 10-�1.8M), and a detection limit of 1.6 x� 10-�6M. The simplified fabrication by a one-step drop casting makes miniaturizing SC-ISEs and fabricating sensor arrays easier to achieve.
MeasureNet
The MeasureNet MCAN® (Multi-functional Chemical Analysis Network) system consists of up to fifteen measurement workstations networked together and managed by a single MCAN® Controller and PC. Each Workstation has two +/- 2.5v analog input channels and one high-speed serial communication channel. The analog inputs are sampled by a two channel high-resolution 24-bit Sigma-Delta A/D. Sigma-Delta converters are designed for direct connection to sensors with low signal levels. The built-in signal conditioning and noise reduction of Sigma-Delta data converters makes them ideal for the low level noisy signals often found in potentiometric measurements of high impedance sensors like pH, ISE and other electrochemical sensors. The high-speed serial channel is for sensors with digital outputs.
The MCAN® workstation measurements are displayed in real-time on the workstation LCD and/or streamed in real-time to MeasureNet's LabKonnect cloud server for storage. Data collected at the workstations can be stored locally on the system PC and/or in cloud data storage accounts. Cloud data can also be monitored in real-time from any internet connected device - computer, tablet or smart phone, allowing the researcher to follow the progress from virtually anywhere while running experiments for extended time periods. LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if measurements go beyond that range. Researchers no longer need to spend valuable time and resources babysitting experiments.

The MeasureNet MCAN® (Multi-functional Chemical Analysis Network) system consists of up to fifteen measurement workstations networked together and managed by a single MCAN® Controller and PC. Each Workstation has two +/- 2.5v analog input channels and one high-speed serial communication channel. The analog inputs are sampled by a two channel high-resolution 24-bit Sigma-Delta A/D. Sigma-Delta converters are designed for direct connection to sensors with low signal levels. The built-in signal conditioning and noise reduction of Sigma-Delta data converters makes them ideal for the low level noisy signals often found in potentiometric measurements of high impedance sensors like pH, ISE and other electrochemical sensors. The high-speed serial channel is for sensors with digital outputs.
The MCAN® workstation measurements are displayed in real-time on the workstation LCD and/or streamed in real-time to MeasureNet's LabKonnect cloud server for storage. Data collected at the workstations can be stored locally on the system PC and/or in cloud data storage accounts. Cloud data can also be monitored in real-time from any internet connected device - computer, tablet or smart phone, allowing the researcher to follow the progress from virtually anywhere while running experiments for extended time periods. LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if measurements go beyond that range. Researchers no longer need to spend valuable time and resources babysitting experiments.
Comparison of the Effects of Biofouling on Voltammetric and Potentiometric Measurements
Kuhlmann, J., Dzugan, L. C. and Heineman, W. R. (2012), Comparison of the Effects of Biofouling on Voltammetric and Potentiometric Measurements. Electroanalysis, 24: 1732–1738. doi: 10.1002/elan.201200194
Abstract
Biofouling of sensors is a common problem when measuring biological samples. The adherence of proteins and biomolecules, called hemostasis, is the first of four steps that lead to biofouling and eventually a foreign body response. This typically occurs within the first hours after the exposure of the biosensor to a biological sample. The purpose of this study was to assess the effect of this initial step of biofouling on cyclic voltammetry and potentiometric measurements. The results show that biofouling occurred rapidly within minutes and strongly affected cyclic voltammetry measurements, while were minimally affected even after 24 hours.
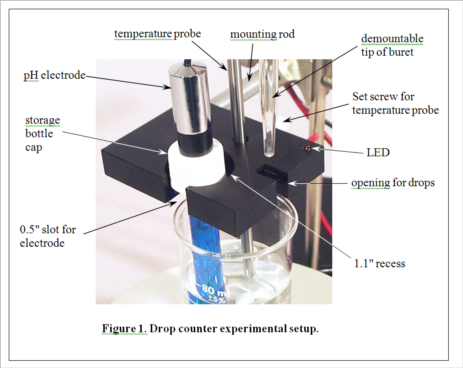
Development of MeasureNet’s optical drop counter began in 2000. Our goal was not only to automate, simplify and shorten the time for pH titration experiments, but also to improve the accuracy of students’ measurements. During its development, various innovative features were added to the design, as seen in Figure 1. MeasureNet’s Multi-Functional Drop Counter holds both a pH probe and a temperature probe, reducing the hardware needed to set up pH titration experiments, and the geometry was optimized to allow the use of small beakers and samples.
Before the days of optical drop counters, simple wire electrical conductivity devices were the only automated and economical solutions for pH titrations in the teaching laboratory. A drop from the buret would make contact with two bare wires positioned closely together, creating a conductive current path. An electronic circuit would then convert this current into a pulse that was counted. A student could then calculate volume based on the number of drops and the average drop size, determined in a separate experiment. The MeasureNet system automatically determines the average drop size in each titration.
The most common method of performing pH titrations is the manual method. The drawbacks to this method:
Time consuming, giving students time to perform few titrations in a lab period.
-
Larger reagent volumes required, making titrations more expensive in terms of reagent usage and disposal.
-
Requires repetition for students to master endpoint detection with reasonable precision.
The 22nd BCCE at Penn State University marks the 10th anniversary of MeasureNet Technology’s introduction of the Optical Drop Counter to the chemical education community. During this decade, the MeasureNet drop counter has become a star in chemistry labs around the country and the world, performing a variety of functions even its designers had never imagined. MeasureNet’s drop counter allows students to leap past the typical pH titration experiment and engage in other interesting and educational titration methods like thermometric, potentiometric, amperometric and colorimetric titrations.
MeasureNet Technology's introduction of the industry’s first Multi-Functional Optical Drop Counter technology in 2002 at the 17th BCCE at Western Washington University obviously caught the attention of the conference attendees and venders. The MeasureNet drop counter has emerged as the industry standard, copied by all its competitors, such as Vernier Software & Technology, Pasco Scientific and MicroLab Inc. MeasureNet is both flattered by this attention and inspired to do more to bring innovative technology into the teaching laboratory.

American students are falling into a giant chasm between the classroom and the workplace.
For three days in 2012, some of the biggest and brightest stars of business and education gathered in Dallas to explore the gap between the types of technical jobs that modern industry has to offer and the ability of workers to perform those tasks. As it stands now, workers simply do not have the advanced skills that employers need. High paying job positions go unfilled, leaving empty holes where a worker should be.
The STEM Solutions 2012 Summit tried to fill in the holes and build pathways that allow students to move more freely from the classroom to the STEM-rich workplace. During the summit, NMSI President & CEO Dr. Mary Ann Rankin encouraged listeners to accelerate STEM education in a panel discussion entitled, “The Supply Side: Education and America’s STEM Future.” The panel looked at various ways to produce enough STEM graduates to satisfy corporate hunger.
Summit participants agreed it is especially important to fill those positions with women.
STEMConnector’s new publication, 100 Women Leaders in STEM, featured Dr. Rankin. Women make up 48 percent of the workforce, yet hold only 24 percent of U.S. STEM jobs. Fewer than 15 percent of American engineers are female.
In another panel discussion, Dr. Rankin focused on defining, understanding and closing the gap between the skills needed by corporations and those offered by today’s universities.
This gap begins as small crevices in early education. Student brains stagnate through boring lectures and memorize facts instead of participating in hands-on education, known to excite young minds about science, technology, engineering and math, or STEM. This stagnation shows in dismal STEM scores. Barely 18 percent of high school seniors perform at proficient levels in science courses.
Weak STEM skills results in poor job prospects and low pay. A student without a solid understanding of STEM subjects cannot develop the technical skills necessary to participate in today’s job market. Employees with poor technical skills will ultimately fail in the modern, highly technical workplace.
Summit participants identified gaps between institutions, with each school adhering to slightly different standards and teaching methods. For example, a kid from one elementary school uses microscopes in the classroom while a child who attends school across town does not. These students eventually merge into a high school science class; the gap between the student with hands-on experience and the one without becomes clear quickly. This gap grows each time the students move from one institution to another, from elementary school to graduate school.
The summit called on educational institutions to fill in the gaps between elementary school, high school, college and graduate school. Many students fall into these gaps and away from careers in STEM fields. Improve each educational segment’s shared understanding of what is necessary to be “college-ready.”
MeasureNet is a prime example of educational technology that actually closes the gap between students and high paying jobs. MeasureNet puts real-world tools into the hands of students of all ages. Instead of sleeping through boring lectures, students use the same inquiry-based chemistry labs, environmental chemistry, instrumental methods, STEM and biochemistry procedures as professional scientists. MeasureNet helps students leap across the STEM chasm and land onto a great-paying job.

WVXU Podcast link by Ann Thompson: Focus on Technology: Bugs Cleaning Wastewater
Cincinnati scientists are engineering special bugs that will clean wastewater and create energy. Ann Thompson takes you into the lab where this is happening in Focus on Technology.
By Ann Thompson
MeasureNet Research Applications
MeasureNet is renowned for putting cutting-edge technology into the hands of students but MeasureNet may someday be famous for helping scientists find new and innovative ways for dealing with the world’s most difficult problems.
The technology provided by MeasureNet combines a high-resolution measurement workstation with their LabKonnect cloud-based software to create a system that allows researchers to monitor their experiments from anywhere. Researchers access information from the cloud, important in experiments lasting for a week or longer.
LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if data goes beyond that range. Scientists no longer spend valuable time and resources babysitting experiments.
This technology was put through its paces recently, when MeasureNet teamed with Dr. Dan Hassett, who creates special bugs that will clean wastewater and create energy. Hassett, molecular genetics professor at the University of Cincinnati, thinks he has found a way to convert sewage into clean water and energy.
Wastewater has stored up energy in the form of pollutants. Hassett is developing bacterial robots, or “bactobots,” that break down these pollutants and release the energy. Sewage treatment plants become biological fuel cells that produce both clean water and energy.
The bactobots are tiny, only about three microns long, but they generate about 400 milivolts with fluctuations as high as 700 milivolts as they clean the water. Hassett increases the amount of power a bactobot can generate through a series of genetic mutations. Measuring the output of these miniscule bacteria is a big job, and that’s where MeasureNet steps in.
MeasureNet helped Dr. Hassett monitor the voltage and current output of biological fuel cells. Typically, the ouput voltage fluctuates and over the course of four days. Thanks to the sensitive measuring equipment and cloud capabilities provided by MeasureNet technology, Hassett found the existence of a second particular bacterium actually increased output over those four days.
While the output from a single bactobot is small, the impact of these biological fuel cells could break one of humanity’s most vexing vicious cycles. Wastewater treatment plants are the single largest consumer of energy, and the second largest user of water is energy production. Introducing a bacteria that would simultaneously clean water and produce energy would be monumental.
MeasureNet is at the forefront of hands-on laboratory technology, both in the classroom and in the research lab. Like technology itself, MeasureNet continuously develops new ways to enhance the lives and learning of students, scientists and everyday people.

"Benzene"
September 20, 2006
Friedrich August Kekulé (1829-1896). German chemist. Initially a student of architecture, Kekulé studied chemistry under Liebig at Giessen and under Dumas in Paris. After brief periods in Switzerland and England (1854), and at the University of Heidelberg (1856), he became Professor of Chemistry at the universities of Ghent (1859) and Bonn (1867-1896). Acknowledged as one of the founders of classical molecular structure theory, Kekulé is best known for his proposal (along with the Scottish chemist, Archibald Scott Couper) that tetravalent carbon can undergo self-linkage to form homocatenated chains and rings (1858), and for his hexagonal cyclic structure for the benzene molecule (1865).
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
Linus Pauling (1901-1994). American chemist. Professor at the California Institute of Technology (1927-1962), and Nobel Prize winner for both chemistry (1954) and peace (1962). Though Pauling began his career as a crystallographer, he is best known for his work on the electronic theory of the chemical bond, as summarized in his 1939 monograph, The Nature of the Chemical Bond, and for his later work on the molecular and electronic structures of large biomolecules. His name is most often associated with his scale of ionic radii (1927), his rules for rationalizing the most stable structures for complex ionic crystals (1929), his extensive use of the both the hybridization (1931) and resonance concepts (1932) in chemical bonding, and his thermochemical electronegativity scale (1932). He also gained considerable notoriety for his political activism in support of nuclear disarmament, and for his controversial advocacy of the supposed medical benefits of megadoses of vitamin C.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

According to the American Chemical Society, hands-on activities significantly enhance learning at all levels of science education. The American Chemical Society, or ACS, is the world’s largest association of individual chemical scientists and engineers, and its newest science education policies communicate the importance of hands-on learning in the science lab. ACS education programs begin pre-kindergarten and extend through undergraduate and graduate studies.
Hands-on activities are the basis for the laboratory portion of any science class and are essential for learning chemistry. A student enrolled in a hands-on chemistry course directly experiences laboratory chemicals, chemical properties and reactions as well as gaining familiarity with laboratory equipment and apparatus. There is simply no substitute for the real-life experience of hands-on training.
One good example demonstrating the superiority of hands-on learning over simulation or lecture is teaching a child to ride a bicycle. Putting a child on a bike and giving her a push teaches her much more in one minute than she could gain by watching videos of other children on bicycles or listening to a lecture on physics. Hands-on learning allows for deeper comprehension of scientific principles, and it benefits the American Chemical Society and the rest of the United States to campaign for a return to this inclusive style of education.
ACS has good reason to promote hands-on learning. A 1982 meta-analysis of 15 years of research including 57 studies of 13,000 students showed that students who participated in hands-on education scored 20 percent better than did students using traditional or textbook approaches. The students engaged in hands-on learning demonstrated gains in creativity, attitude, perception and logic. The National Assessment of Educational Progress noted that teachers who incorporated hands-on activities into the curriculum at least once a week out-performed their peers by more than 40 percent of a grade level in science.
No Child Left Behind act, or NCLB, appears to have had an unintended negative impact on hands-on learning, particularly for children at the elementary and middle school ages. Science is not a federally mandated assessment, so teachers and administrators instead focused on subjects compliant with NCLB assessments such as reading and math. Pupils who were students during the height of NCLB continue to struggle with this deficit well into their post-secondary education unless given an opportunity to catch up with adequate hands-on training in the laboratory. For many, this chance does not occur until high school or beyond, if at all.
Undergraduate and graduate institutions must offer hands-on opportunities in the laboratory to ensure graduates are able to meet and overcome the challenges of modern chemistry. Some of these advanced students will rely on these skills to solve real-world problems in the workplace while others return to the classroom to teach others. ACS challenges teachers to reach new goals of excellence and advocates certain measures to help educators achieve superiority, including requiring teachers to take undergraduate courses to ensure they are prepared to teach coursework and enhancing funding at all levels so that science teachers have access programs that allow them to expand and update their science knowledge base. ACS also encourages teachers and school systems to use technology to reach students with different learning styles. The ACS is also dedicated to improving the work conditions of science teachers, reduce attrition and improve safety in the classroom laboratory.
Some educators are tempted to take advantage of the shortcuts modern technology has to offer but teachers should choose wisely. Computer simulations are flashy and inexpensive but are not an adequate substitution for hands-on activities, even at the collegiate level. Educators must use computer simulation as a supplement to, not as a replacement for, hands-on learning. While intellectual curiosity should be piqued with internet searches, guided by lectures and accelerated with computer simulation and video, hands-on learning is still one of the most effective ways to instill confidence in knowledge and increased comfort with using laboratory and technical equipment.
ACS recognizes the special need for new assessment equipment at the undergraduate level. This equipment should assess a student’s understanding of science and the use of methods of science to give the instructor a fuller appreciation of the student’s grasp of science, not just his ability to recite scientific facts. Giving students access to a shared diode-array visible or UV-vis spectrometer to perform absorption, emission, fluorescence and reflectance experiments has so much more impact than just describing.
The American Chemical Society emphasizes the importance of a hands-on, inquiry-based approach to science to not only help students gain knowledge and understanding of scientific principles but also to teach students how scientists explore and make sense of the natural world. Hands-on learning teaches students to learn with the same tools used by professional scientists. It teaches them to think like scientists by creating hypotheses, making observations and performing inquiries. Students improve skill proficiency in scientific processes, such as laboratory work, graphing results and interpreting data.
Hands-on learning has been around since the days of Aristotle, who said, “What we have to learn to do, we learn by doing.” Hands-on learning enhances the student’s ability to test, sense, apply and learn. This type of learning involves and improves upon the art of questioning. Use hands-on, state of the art analytical instrumentation to revolutionize the way your students learn chemistry and science in your classroom laboratory.
“I hear and I forget, I see and I remember. I do and I understand”
Chinese proverb