
The modernization of a universities General Chemistry laboratory program can be a daunting and yet necessary task. This is especially true when you have 2000+ students taking General Chemistry every year. The University of Tennessee, Knoxville decided it was time to modernize their General Chemistry Lab Program for them to remain competitive. They knew that Electronic Data Collection Technology needed part of this process, and needed to be integrated into the labs. I am happy to say The University of Tennessee, Knoxville decided MeasureNet's Electronic Data Collection System was the technology selected in the redesign of their General Chemistry Laboratory Program.
“The laboratory makeover not only benefits the first-year students, but will help improve their preparation and success as they go on to take upper-level chemistry courses, “
Al Hazari, Director of Undergraduate Laboratories.
%20Systems-resized-601.png)
Department of Chemistry and Biochemistry, Miami University, Oxford, Ohio 45056, United States
J. Chem. Educ., 2013, 90 (4), pp 500–505
DOI: 10.1021/ed300340x
Publication Date (Web): March 15, 2013
Copyright © 2013 The American Chemical Society and Division of Chemical Education, Inc.
Abstract
The large class sizes of first-year chemistry labs makes it challenging to provide students with hands-on access to instrumentation because the number of students typically far exceeds the number of research-grade instruments available to collect data. Multifunctional chemical analysis (MCA) systems provide a viable alternative for large-scale instruction while supporting a hands-on approach to more advanced instrumentation. This study describes how the capabilities of MCA systems are extended to introduce liquid chromatography (LC) and flow injection analysis (FIA) in undergraduate laboratories. A semi-micro plastic cuvette with a Teflon tubing insert is fashioned as the flow cell for a MCA absorbance–fluorescence detector. Two MCA systems, Vernier and MeasureNet, are used in two unique experiments demonstrating the detection of salicylate in aspirin tablets by FIA and the LC separation of a mixture of riboflavin and fluorescein. Both instruments, composed of a syringe pump, T-injection valve, and the MCA detector, operated in the kinetic mode, are rugged and inexpensive permitting student construction, if desired.
Micro Solid-Contact Ion-Selective Electrode Using a Carbon
Nanotube Tower as Ion-to-Electron Transducer and Conductive
Substrate
Xuefei Guo, Timothy Meyung, Yeoheung Yun, Vesselin N. Shanov, H. Brian Halsall, William
R. Heineman
Electroanalysis Volume 24, Issue 11 DOI: 10.1002/elan.201200348
Abstract
Solid contact (SC) ion-selective electrodes (ISEs) have been recognized as the next generation of ISEs. In this work, the electrical conductivity and mechanical strength of a carbon nanotube (CNT) tower enable it to play the dual roles of transducer and substrate for micro SC-ISEs. The electrode had a close to Nernstian slope of 35 mV/decade aCa2+, a linear range of four orders of magnitude of calcium ion activity (10�-5.6 to 10-�1.8M), and a detection limit of 1.6 x� 10-�6M. The simplified fabrication by a one-step drop casting makes miniaturizing SC-ISEs and fabricating sensor arrays easier to achieve.
MeasureNet
The MeasureNet MCAN® (Multi-functional Chemical Analysis Network) system consists of up to fifteen measurement workstations networked together and managed by a single MCAN® Controller and PC. Each Workstation has two +/- 2.5v analog input channels and one high-speed serial communication channel. The analog inputs are sampled by a two channel high-resolution 24-bit Sigma-Delta A/D. Sigma-Delta converters are designed for direct connection to sensors with low signal levels. The built-in signal conditioning and noise reduction of Sigma-Delta data converters makes them ideal for the low level noisy signals often found in potentiometric measurements of high impedance sensors like pH, ISE and other electrochemical sensors. The high-speed serial channel is for sensors with digital outputs.
The MCAN® workstation measurements are displayed in real-time on the workstation LCD and/or streamed in real-time to MeasureNet's LabKonnect cloud server for storage. Data collected at the workstations can be stored locally on the system PC and/or in cloud data storage accounts. Cloud data can also be monitored in real-time from any internet connected device - computer, tablet or smart phone, allowing the researcher to follow the progress from virtually anywhere while running experiments for extended time periods. LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if measurements go beyond that range. Researchers no longer need to spend valuable time and resources babysitting experiments.
The folks here at MeasureNet have been hard at work integrating new probes into our system and creating new experiments. In this blog entry we'll be introducing the following new experiments and probes:
- Dual Probe Experiments (Pressure, Temperature & Voltgage)
- Thermocouple Temperature Probe
- Melting point Experiment
Dual Probe Experiments
MeasureNet now has the ability to collect data from 2 of the same probe if using the voltage, pressure, or temperature probes. New experiment options have been added to each probe's menu to allow dual probe collection. In order to use the dual probe option, customers will need the MeasureNet dual probe adaptor (pictured below).

Thermocouple Temperature Probe
MeasureNet also offers a thermocouple type J temperature probe now for applications that fall outside the range of our standard temperature probe. The new thermocouple probe has a temperature range from -180C to +475C.
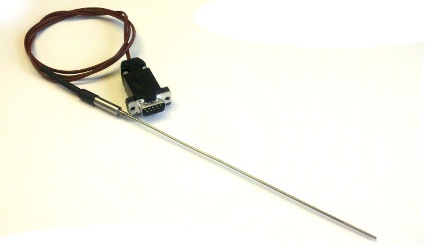
Mel-Temp Switch & Experiment
Along with the thermocouple probe, MeasureNet now offers a solution that will make Melt Temp experiments easier. During a Mel-Temp experiment, you can use the Melt Temp Switch to keep track of a small range of temperatures when your compound nears melting temperature. You can then use print code 400 to print an overall temperature graph along with a graph that focuses on the range where the sample started to melt.

Make sure to keep a lookout for the next set of probes and experiments. We still have more to show.

The MeasureNet MCAN® (Multi-functional Chemical Analysis Network) system consists of up to fifteen measurement workstations networked together and managed by a single MCAN® Controller and PC. Each Workstation has two +/- 2.5v analog input channels and one high-speed serial communication channel. The analog inputs are sampled by a two channel high-resolution 24-bit Sigma-Delta A/D. Sigma-Delta converters are designed for direct connection to sensors with low signal levels. The built-in signal conditioning and noise reduction of Sigma-Delta data converters makes them ideal for the low level noisy signals often found in potentiometric measurements of high impedance sensors like pH, ISE and other electrochemical sensors. The high-speed serial channel is for sensors with digital outputs.
The MCAN® workstation measurements are displayed in real-time on the workstation LCD and/or streamed in real-time to MeasureNet's LabKonnect cloud server for storage. Data collected at the workstations can be stored locally on the system PC and/or in cloud data storage accounts. Cloud data can also be monitored in real-time from any internet connected device - computer, tablet or smart phone, allowing the researcher to follow the progress from virtually anywhere while running experiments for extended time periods. LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if measurements go beyond that range. Researchers no longer need to spend valuable time and resources babysitting experiments.
Comparison of the Effects of Biofouling on Voltammetric and Potentiometric Measurements
Kuhlmann, J., Dzugan, L. C. and Heineman, W. R. (2012), Comparison of the Effects of Biofouling on Voltammetric and Potentiometric Measurements. Electroanalysis, 24: 1732–1738. doi: 10.1002/elan.201200194
Abstract
Biofouling of sensors is a common problem when measuring biological samples. The adherence of proteins and biomolecules, called hemostasis, is the first of four steps that lead to biofouling and eventually a foreign body response. This typically occurs within the first hours after the exposure of the biosensor to a biological sample. The purpose of this study was to assess the effect of this initial step of biofouling on cyclic voltammetry and potentiometric measurements. The results show that biofouling occurred rapidly within minutes and strongly affected cyclic voltammetry measurements, while were minimally affected even after 24 hours.
Sparking a student’s interest in science, technology, engineering or math can launch her on a new career in the highly-lucrative and rapidly-expanding opportunities in STEM fields. Great pay, interesting work, job security and good working conditions are the norm for those holding a STEM degree or working in a STEM field, such as computer and information technology, engineering or life science. STEM fields are closing the gender gap, encouraging more women to reap the benefits of an education in science and technology. Solid STEM training benefits the workers of today and tomorrow.
Good Pay
Whether you earned a degree in a STEM field or you work in a STEM field without holding a diploma, STEM increases your average wages. A STEM worker with a bachelor’s degree will earn on average $7 more per hour than another person with the same credentials in a non-STEM occupation. Just holding a degree in a STEM field will increase wages. A person with a STEM degree makes 13 percent more doing the same job as a person without such an education. Those who study STEM or gain a degree in a STEM field have higher wages, even if they don’t ultimately work in a STEM field.
To put it in hard numbers, the Bureau of Labor Statistics, or BLS, reports the average annual wage for all STEM occupations was $77,880 in May of 2009. Natural science managers were the highest paid STEM workers, followed by engineering and computer science managers. These highest-paid workers held mean wages in excess of $100,000 or more.
Interesting Work
Working in a STEM job is exciting. All the cool things we love today are the result of the innovating and creative thinking of countless STEM workers, including our phones, computers and video games, live-saving medicines and other technical miracles. Whether a STEM worker uses her skills to bring a rare species of animal back from the brink of extinction or to design the automobile of tomorrow, work in a STEM field will always be challenging and highly rewarding.
Lower Unemployment
Workers in STEM jobs face a lower risk for unemployment than laborers in other fields. In 2010, unemployment rates among non-STEM workers were almost 10 percent, compared to only 5.3 percent unemployment among STEM workers. It is possible that this lower unemployment rate is due to the higher level of education among STEM workers; solid education, especially in STEM fields, reduces unemployment.
Increased Opportunities
The topic of STEM jobs and the education to get those lucrative jobs has never been hotter. The BLS projects STEM job growth to have grown 22 percent between the years of 2004 and 2014. Computer specialist occupations are expected to have grown much faster than average. Despite a sluggish national and global economy, STEM jobs continue to enjoy expanding opportunities for well-educated workers.
Decreased Gender Gap
There is a smaller economic gender gap in STEM jobs than in other professions, with women earning nearly the same income as men for performing a STEM job. Women who work in STEM fields make on average 33 percent more than women who work in non-STEM jobs. Many women with a STEM degree work in education or healthcare rather than in a STEM field.
Good Working Conditions
STEM jobs are usually performed inside air-conditioned offices or laboratories in technical parks located in nice sections of the country, such as southern California or Boulder, Colorado. Most of these jobs are quite safe, extremely exciting and always challenging. STEM workers typically share clean, quiet environments with other well-educated, like-minded professionals.
Before a worker enters the profitable and rewarding STEM field, he needs a solid STEM education. Early and secondary education is the key, whether a student plans to study STEM or enter a STEM field directly out of high school. Introducing students to science, technology, engineering and math at an early age can foster a passion that puts future workers on the right path, rich in financial and professional rewards.
http://educationupdate.com/archives/2011/NOV/HTML/col-stemjobs.html
http://www.bls.gov/opub/ooq/2007/spring/art04.pdf
http://www.bls.gov/opub/mlr/2011/05/art1full.pdf
http://www.esa.doc.gov/sites/default/files/reports/documents/womeninstemagaptoinnovation8311.pdf

President Barack Obama hosts the second White House Science Fair celebrating the student winners of a broad range of science, technology, engineering and math (STEM) competitions from across the country. The President talked with Samantha Garvey, 18, of Bay Shore, N.Y., about her environmental sciences project examining the effect of physical environment and predators on a specific species of mussel, in the State Dining Room of the White House, Feb. 7, 2012. (Official White House Photo by Pete Souza)
Administration and Private Sector Announce over $100 Million in Commitments and Additional Steps to Prepare 100,000 New Science, Technology, Engineering and Math Teachers
Shots were fired in the State Dining Room on February 7, 2012, much to the delight of President Obama and the school children attending the second White House Science Fair. Joe Hudy grabbed hold of his Extreme Marshmallow Cannon and blasted a pair of curtains with extreme prejudice after allowing the president to pump up the air-powered canon. The President added power to the science project before placing the technology back into 14-year-old Arizona student’s hands. During the science fair, President Obama announced new steps aimed at putting today’s powerful technology directly into the hands of students in hopes of helping young people do better in science, technology, engineering and math, collectively known as STEM.
President Obama has issued a national challenge to prepare 100,000 qualified STEM teachers within the next decade. These teachers will then, in turn, help one million students graduate with degrees in STEM fields within the next ten years.
The first step to achieving these goals included a request for $80 million for teacher preparation in the fiscal 2013 budget, sent to Congress on February 13th, 2012. This money will support effective STEM teacher training programs, such as those that allow a student to earn a teaching certificate along with a degree. Another $22 million comes from pledges by philanthropic and private sector organizations. More than 115 organizations immediately responded to President Obama’s call to action by creating a group called “100Kin10.” Fourteen of these organizations, including the Carnegie Corporation, Google and Bill & Melinda Gates Foundation, announced a $22 million fund intended to prepare and support STEM education. The future of these organizations rely on a highly educated workforce and are dedicated to improving the skills of future workers by putting state-of-the-art technology directly into the young hands of aspiring scientists.
The 100Kin10 partners have also made more than 100 individual commitments, including Race to the Top competition. President Obama believes that improving STEM education relies heavily on systemic reform on the state and local level and that these reforms should expand opportunities for hands-on learning. The Department of Education will focus on STEM criteria during the Race to the Top competition as a way to improve students’ achievements and interest in STEM studies.
It is imperative that educators put current technology into the hands of students. Students have a natural curiosity about cutting-edge technology but sometimes lose interest because the student does not connect exciting STEM careers with the boring, outdated equipment he uses in his school laboratory. Educators have the unenviable task of making this connection for the student. Products such as the Measurenet MCAN Laboratory Electronic Data Collection system makes this job easier by ensuring students are using the same technologies in the school laboratory as used in postsecondary and professional applications.
Industry leaders agree that a hands-on approach in secondary and postsecondary education will improve the standing of American companies engaged in global economic competition. Practical experience with technology and instrumentation is a valuable asset, both to students planning to go to college and to those entering the workforce directly from high school.
The White House and its partners are aiming higher when it comes to preparing students for future jobs in science, technology, engineering and math. The administration and the private sector are firing back against competitors in the global market by arming highly qualified instructors with the finest educational supplies and techniques, and by building new pathways between teachers and students. Perhaps the best ammunition for the Extreme Marshmallow Cannon was the teacher who put today’s cutting-edge technology directly into Joe Hudy’s hands.
Science and technology bond together like carbon and hydrogen. One field benefits the other- scientific discoveries advance technological applications which then return more sophisticated research tools to the scientific community. Many of your chemistry students will graduate into a world that integrates science and technology into a singular platform that utilizes electronic data collection technology, a safer and more efficient mode of gathering and managing information. Give your freshman chemistry students the technological edge they need to facilitate learning while simultaneously freeing yourself from expensive hardware upgrades, viruses and archaic forms of monitoring student progress in your freshman chemistry lab.
When you switch to electronic data collection technology, you’ll immediately notice how much more available space you have in your general chemistry lab. In the typical, old-fashioned general chemistry lab, each student shared bench space with their own large, cumbersome PC. These PCs are at increased risk for virus infection, costly hardware and software upgrades and usually need to be replaced every three to four years. The MeasureNet MCAN, or Multifunctional Chemical Analysis Network, replaces up to 15 individual PCs with a single PC that is used by the instructor to monitor student activity and manage their data files, locally or on the cloud.
Student workstations integrate with a wide variety of probes and other chemistry laboratory apparatus that enable your chemistry lab students to accurately perform hands-on experiments in general chemistry, environmental chemistry, STEM and biochemistry labs. These hands-on lab exercises are critical for the students’ development of basic chemistry concepts.
Chances are good that students in your general chemistry lab are already technologically advanced and have used electronic data collection technology in high school. They are also quite accustomed to cloud computing from using products like Google Docs and Dropbox, where both software and files are saved online rather than on a personal computer. MeasureNet’s MCAN technology merges electronic data collection and cloud computing capabilities together. MCAN allows your student to measure and collect high resolution data in the lab and store it on the cloud for later analysis and lab report generation. Students, especially science students, will be excited to use the advanced technology MeasureNet MCAN offers. MeasureNet MCAN technology takes your students to the next level, giving your students the edge they need when whether they go on to industry or pursue advanced degrees in science.
Cloud computing also helps you monitor your chemistry lab students from the instructors PC to be sure they are conducting the experiment, collecting data and analyzing information properly. You can also monitor live data collection experiments remotely. Using the internet you can connect to MeasureNet MCAN workstations from outside the chemistry lab using a computer, tablet or smart phone.
MCAN electronic data collection technology can easily be in integrated into your current chemistry lab curriculum and can be adapted to fit a variety of teaching styles.
- POGIL
- STEM
- Self Directed
- Verification-style
- Inquiry-based Chemistry Labs
- Hands-on learning
You can easily integrate MeasureNet based experiments into your current lab curriculum. Here is a small sample of experiments that can be conducted with the MCAN technology.
- Gas Laws
- Colligative Properties
- Enthalpy of Reaction — Hess's Law
- Determination of the Heat of Neutralization of a Variety of Strong Acids and Bases
- Chemical Kinetics
- Determination of a Reaction Equalibrium Constant Using Absorption Spectroscopy
- pH and Buffer Solutions
- pH titrations and end-point determination using Drop Counter
- Identifying a Weak Unknown Acid
- Determination of the Molecular Weight of a Volatile Liquid Using the Ideal Gas Law
- Vapor Pressure and Heat of Vaporization
Using MeasureNet’s MCAN technology in your lab means you’ll spend less time working as a computer repair technician and more time teaching chemistry. The MeasureNet MCAN frees you from problems usually associated with PC-based systems. Virus removal and reimaging computers will be a thing of the past.
Get back to doing what you love – teaching chemistry to hungry minds – by replacing your old, worn out computers with intuitive space saving MCAN electronic data collection technology. Excite students in your general chemistry labs by using the same technically advanced instrumentation used by university research labs and real-world industry chemistry labs. Make your chemistry lab program exciting and technologically relevant to students by using electronic data collection technology in your labs.

The MCAN® Concept
The MeasureNet Multifunctional Chemical Analysis Network (MCAN® ) is an innovative analytical instrumentation eliminating a multitude of the obstacles that are associated with the PC-based lab systems. With the consolidation of student data acquisition workstations into a solitary network for each group of students, expensive hardware upgrades, computer viruses and the footprint of large equipment is eliminated.
The student workstations we provide interface with numerous kinds of probes as well as other apparatus that are utilized in chemistry laboratories. This allows for a broad range of general chemistry, physical chemistry and biochemistry lab experiments.
MeasureNet can be useful in a freshman chemistry lab or in advanced chemistry labs. Whether used in the freshman chemistry lab, biochemistry, STEM, or environmental chemistry laboratories, MeasureNet is an essential tool.
With MeasureNet, the student takes the measurements at their workstation and the results of those measurements can be stored and then monitored on a single central computer. This allows the instructor to follow student progress and data files. There is no need to contend with the multiple headaches that are associated with managing student lab data because MeasureNet can do it all for you.
Solutions in the Chemistry Lab with MeasureNet
- Less bench space due to energy efficient workstations with smaller foot prints.
- Securely Protected online data storage for students.
- Viruses and computer re-imaging are things of the past with MeasureNet.
- The collection of data electronically allows for more time being spent on the actual experiments.
- Eliminates the need to upgrade large numbers of computers every few years.
- The best quality, research grade chemistry probware is utilized to collect high-resolution data.
General Chemistry Lab Experiments Performed with MeasureNet
MeasureNet was designed to offer instructors considerable academic flexibility with its ability to support a wide range of experiments.
MeasureNet has the ability to support experiments for individual or collaborative student projects in a freshman chemistry lab or in an advanced lab.
The adoption of electronic data acquisition does not require the disposal of experiments that have been proven to build the students’ skills or enhance their understanding of imperative concepts. The longstanding experiments can very easily be integrated into curricula combined with the experiments appropriate for MeasureNet. Other experiments requiring conversion can be modified with the assistance of our curriculum specialists.
Title examples for Guided inquiry, Self-Directed, POGIL, Verification-style and STEM experiments utilizing MeasureNet are as follows:
- Specific Heat of a Metal
- Hot & Cold Packs- Dystan Medical Supply Company
- A Colligative Property of Solutions-Freezing Point
- Quality control at the GlassEX Company-Self-Directed
- Buffer & pH Solutions
- Proteins & Amino Acids
- Chemical Kinetics
- Gas Laws
- Voltaic Cells
- Substances Specific Heat
- Analysis of Metals Emissions
- Heat of Vaporization & Vapor Pressure
- Determining Chromium (VI) Concentrations using Absorption Spectroscopy
- Colligative Properties
- Determining the Cause of a Fish Kill Located in the Clark Fork of the Columbia River
- Identification of a Weak Unknown Acid
- Analysis of the Phosphorus in Cola
- Identification of an Unknown Metal-Self Directed
- Determining the Ka Value of a Weak Acid
- Determining the concentration of Acetic Acid in Vinegar
- Determining the Molecular Weight, with the use of the Ideal Gas Law, of a Volatile liquid
- Hess’ Law-Enthalpy of Reaction
- Analysis of the Phosphorus in Fertilizer
- Determining the Heat of Neutralization for Various Strong Bases & Acid
- Reaction Stoichiometry & Moles
- Determining a Reaction Equilibrium Constant with the Use of Absorption Spectroscopy
- Analysis of Emission of Aequeous Solutions from Group IA & IIA Metal Salts
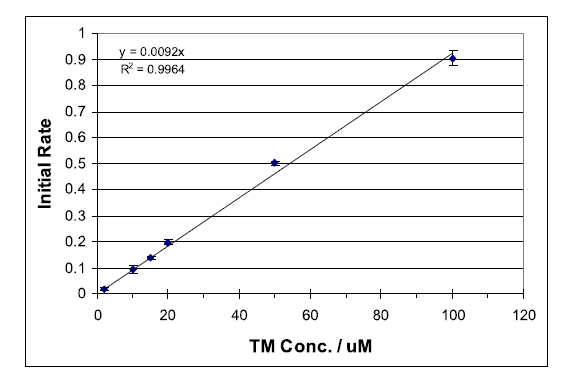
Measurement of molecular fluorescence is an important analytical technique in chemical and biological sciences. The capacity of detecting very small fluorophore concentrations, or small changes in its concentration, combined with high specificity make this technique a very powerful analytical tool.
Kinetic methods for determining reaction rates are commonly used. While most experiments are designed to determine the order of a particular reaction in order to gain insight to the reaction mechanism, kinetic methods are also used for quantitative analysis. Determination of the initial reaction rate is one way of quantitatively analyzing a compound within a suitable reaction system.
The following experiment is designed to introduce students to both of these concepts, fluorescence and kinetics as analytical method. This combined technique has the advantage of increased analyte specificity over equilibrium-based fluorescence measurements. As only the compound that is reacting is causing a change in the measured fluorescence signal, steady-state interferences are largely eliminated.
Thiamine (vitamin B1) is essential for the metabolism of carbohydrates and normal function of he nervous and cardiovascular systems. Severe vitamin B1 deficiency will eventually lead to beriberi, characterized by abnormal functions of the muscular and nervous systems, as well as heart and brain abnormalities. Vitamin B1 occurs naturally in foods like whole grains, nuts, vegetables, pork, and liver.
Thiamine (TM), a non-fluorescent compound, has been found to be oxidized selectively by mercuric oxide (HgO) at a rate suitable for monitoring with standard fluorescence spectrometers.1 The oxidation product, thiochrome (TC), is fluorescent with a strong absorbance maximum at 367 nm and fluorescence emission at 444 nm. Deprotonation of TM yields a non-fluorescent tricyclic intermediate (CI), which is oxidized to TC as outlined in Scheme 1. Immediate oxidation of CI is essential to avoid the formation of several non-fluorescent productions.1 Thus, it is important to follow the sequential addition of reactants as outlined below... To view the complete experiment Click Here.